As a GMP-compliant compounder of cytostatica, parenteral products, and other infusions, Medios makes a key contribution to reliable patient care. Our process sets standards in quality, safety, and punctuality.
As a pharmaceutical compounder in accordance with Article 13 of the German Pharmaceutical Products Act (AMG).
Patient-specific therapies are a core component in the treatment of complex and chronic diseases. Because every individual application is precisely tailored to the demands of the individual patient, compounding these medicinal products is a particularly demanding task. It requires complex specialist knowledge, first-class technical equipment, and precise compounding to the highest quality and safety standards.
As a pharmaceutical compounder in accordance with Article 13 of the German Pharmaceutical Products Act (AMG), Medios has many years of experience in this field. At our facilities we compound ready-to-use patient-specific therapies from approved medicinal products in the latest cleanroom laboratories. We work strictly in accordance with Good Manufacturing Practice (GMP).
We offer
Our broad-based logistics and distribution system guarantees that our high-quality products get to the pharmacy quickly and safely. Our decentralized compounding and optimized utilization of plant capacities make our operations highly efficient in terms of time and expense. Our offering even includes patient-specific product blistering. We document all compounding steps as well as the entire supply chain for maximum transparency.
With our digital order and billing portal* mediosconnect, we optimize cooperation with medical specialists, health insurers, and pharmacies. In addition, we advise on all pharmaceutical, logistics, commercial, and billing matters.
With our leading Specialty Pharma competence, we have also established ourselves as a key compounding partner for hospitals. We use our expertise to support and inform them on new products – from clinical trials through to marketability. We have a wide range of permits to compound both sterile and non-sterile investigational medicinal products. This enables us to compound both small batches and bulk deliveries equally.
*Ophthalmology

Reliability has the highest priority in the supply of pharmacotherapeutics.
We achieve this by working closely with medical specialists and pharmacists as partners throughout the process, and by applying the high quality and safety standards represented by Good Manufacturing Practice (GMP).
From the prescription through to the use of patient-specific therapies, the entire process is designed to offer our partners maximized safety and efficiency. Specialized pharmacies check the prescriptions for plausibility, place orders with Medios, and deliver the final ready-to-use products to medical specialists. The extended plausibility check carried out by Medios provides additional security. From the request through compounding to provision – all the individual process steps are perfectly coordinated.
(GMP)
GMP stands for systematic quality assurance by means of clearly defined requirements for quality assurance of production workflows and environments in pharmaceuticals manufacturing.
The main GMP criteria are
We confirm that in compliance with the GMP standards, the highest levels of quality and safety are guaranteed for our patient-specific pharmacotherapies. Qualified equipment, validated manufacturing processes, and our trained staff ensure accuracy, purity, and contents of the products.
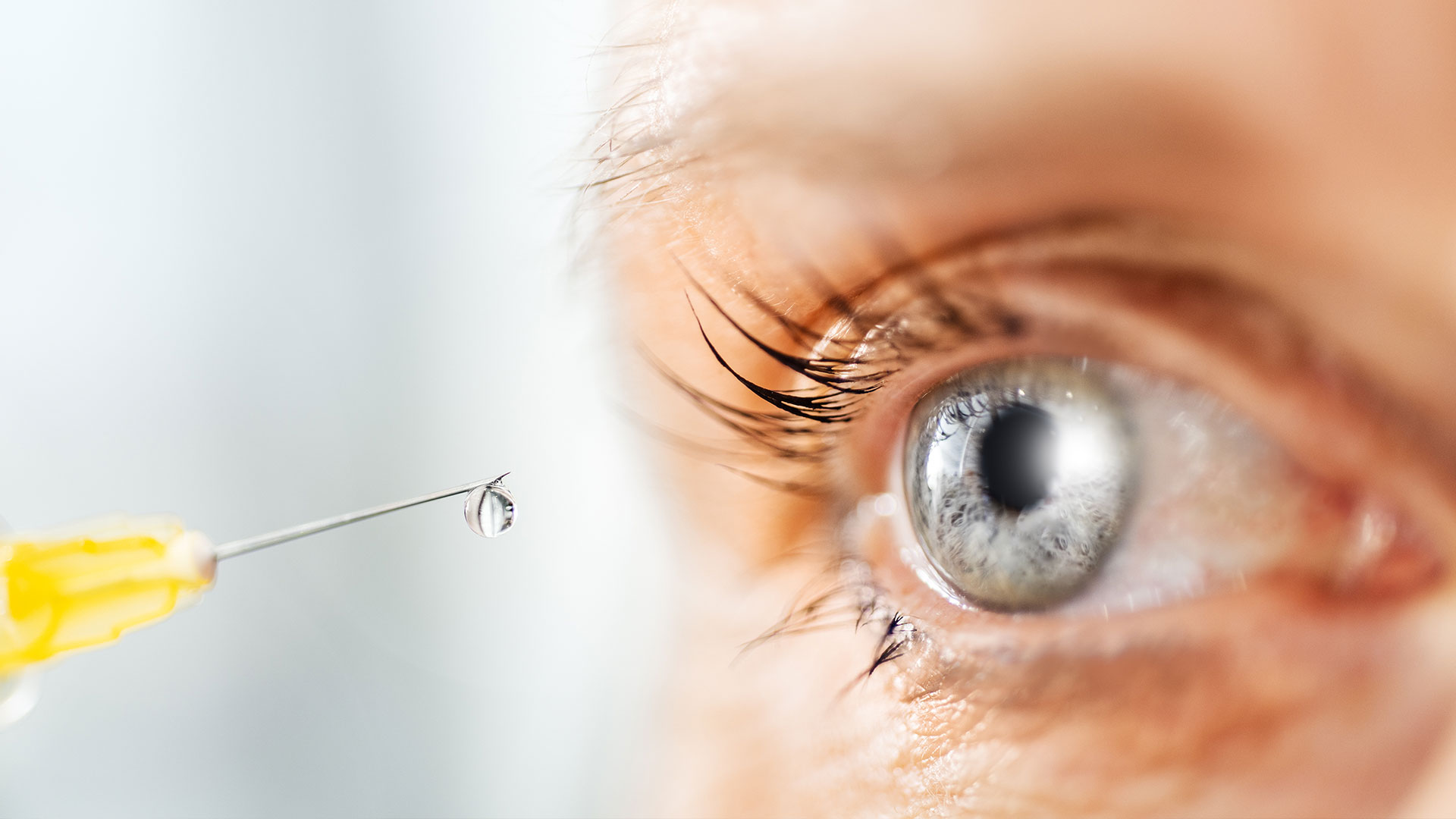
Individual medication for degenerative eye diseases
Ophthalmology is one of the oldest medical specialties and deals with the human eye and eye disorders. In our increasingly ageing society, degenerative eye diseases are of particular importance. For example, age-related macular degeneration (AMD) is the most common eye condition in the western world. It leads to considerable visual impairment in people over the age of 50 and can even cause near blindness in its late stages.
Your benefits
Macular degeneration and related diseases are an important focus of our expertise at Medios. Even though AMD cannot be cured, various therapies can be used to slow down the progression of the disease and preserve vision for longer. To this end, we offer ophthalmology practices and clinics a broad range of supply including the complete range of finished medicinal products for this indication and the production of patient-specific IVOM formulations.
The main aim when reliably compounding high-quality patient-specific infusions for a wide range of different therapy areas is to provide tailored, effective, and well-tolerated medicinal products to everyone with a complex and severe disease. By making sure that every pharmacy in the region can offer medical specialists a supply of patient-specific therapies, we significantly enhance the quality of life for these patients.
Patient-specific therapies are tailored to the individual needs of each patient and are milestones in the ongoing development of individualized medicine.
Do you have any questions about our range of products and services? Would you like to find out how you can partner with Medios?
We look forward to hearing from you!


Medios Competence Network Ophthalmology
We have been informed of a fake listing of a cosmetic product on the TEMU platform in which Medios is falsely named as the distributor. Unfortunately, such unauthorized product listings are repeatedly observed on unreliable platforms like TEMU.
We would like to clarify: This offer is in no way connected to Medios and is not authorized. Our legal department has been informed.
Please obtain medical products exclusively through authorized and verified sources.
If you have any further questions, please contact: ir@medios.group